The chemical reactivity is a qualitative measure for the tendency of materials to form chemical bonds. The question as to the actual sites of high or low reactivity within a given substance is manifest. An answer to the question becomes challenging if these sites are to be identified within a single molecule. In the Experimental Physics 1 / Surface Physics Group at TU Ilmenau led by Prof. Jörg Kröger, in close collaboration with quantum chemist Prof. Marie-Laure Bocquet from the Université Sorbonne, Paris, a molecular probe has now been conceived to answer the question for a single dye on a surface. The results of this research have just been published in the prestigious Journal of Physical Chemistry Letters.
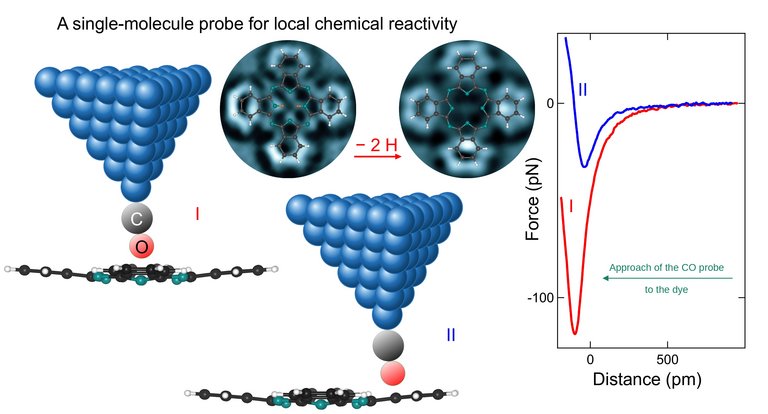
The probe consists of the tip of a modern atomic force microscope that is equipped with a single CO molecule. The CO molecule is controllably picked up by the metal tip from an ensemble of CO adsorbed on the surface prior to the experiments. The O atom represents the outmost apex atom of the functionalized tip. In the next step, the tip is approached to a specific intramolecular site of a single dye, which is a phthalocyanine in the present case. The distance is reduced with picometer resolution down to less than a typical bonding length; that is, the O atom of the molecular probe and the chosen intramolecular atom come as close as a few tenths of a nanometer.
In order to infer the interaction between the sensor and the dye, i.e., the mutual bonding force, the change in the resonance frequency of the rapidly oscillating tip is simultaneously recorded during the approach. The oscillation frequency is approximately 30000 Hz and its amplitude 10 pm. Because of the narrow resonance linewidth, resonance frequency changes on the order of 0.1 Hz can routinely be measured. The distance-dependent resonance frequency change exhibits a pronounced minimum that signals the onset of a chemical bond between the sensor and the studied molecule.
The center of the molecule gives rise to the strongest attraction
The decisive observation in the low-temperature experiments is the spatial variation of the maximum short-range attraction within the single dye: the center of the macrocyclic molecule gives rise to the strongest attraction. This first evidence for a relation between the maximum attraction and the local chemical reactivity was corroborated by exploring a modified dye. The central pyrrolic H atoms were removed atom by atom by the microscope tip in order to artificially enhance the chemical reactivity. The maximum short-range attraction between the CO probe and the new macrocycle center is indeed larger. Prof. Jörg Kröger:
These novel findings open the path to experimentally identify with atomic resolution reactive centers of biologically or chemically relevant molecules. This represents an important step for future nanotechnologies that aim at engineering functional molecules.

Parts of the discussed experiments were performed with the scanning probe apparatus funded by the Federal Ministry of Education and Research within the ForLab project. The continuous supply with liquefied helium from the helium recovery used in the ForLab project was of key importance to the success of the experiments.
Publication in The Journal of Physical Chemistry Letters: "Extraction of Chemical Reactivity and Structural Relaxations of an Organic Dye from the Short-Range Interaction with a Molecular Probe "; Rothe, Karl; Néel, Nicolas; Bocquet, Marie-Laure; Kröger, Jörg, J. J. Phys. Chem. Lett. 2022, 13, 8660-8665.
https://doi.org/10.1021/acs.jpclett.2c02140
*Illustration: the metallic tip of the atomic force microscope carries a single CO molecule, examining different sites (I and II) of an adsorbed dye molecule with atomic resolution for their chemical reactivity. A quantitative measure of local chemical reactivity is the magnitude of the maximum attraction, i.e., the strength of the sensor-dye bond, which appears as a minimum in the force-distance curves.
Contact
Prof. Jörg Kröger
Head of Experimental Physics 1 / Surface Physics Group - Institute of Physics

