Scientists of the group Experimental Physic 1 / Surface Physics at TU Ilmenau led by Prof. Jörg Kröger, in close collaboration with quantum chemist Prof. Marie-Laure Bocquet from the Université Sorbonne, Paris, have succeeded in measuring forces and energies in the metalation of a single dye molecule. The results will appear with the title “Quantifying Force and Energy in Single-Molecule Metalation” in the prestigious Journal of the American Chemical Society, the flagship journal of the American Chemical Society.
Publication in the Journal of the American Chemical Society
Forces involved in the assembly of a single dye molecule
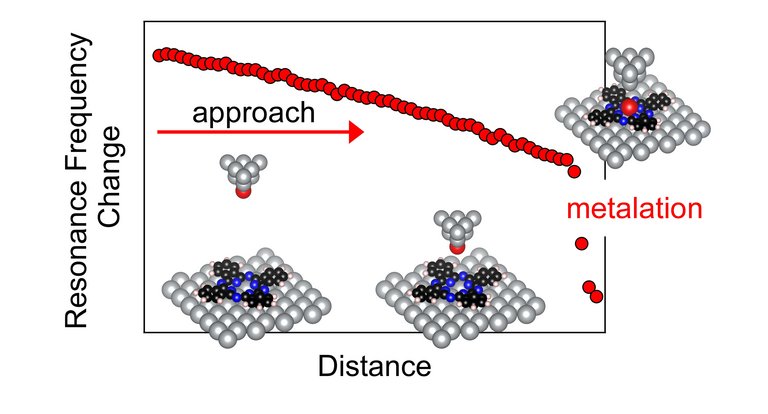
The metallic tip of the atomic force microscope used in the experiments serves several purposes at the same time, the high-resolution imaging of reactant and product of the metalation reaction, the distance-dependent measurement of the attractive interaction between the reactants, and the manipulation of matter at the atomic scale. In the first step, the pyrrolic hydrogen atoms are removed atom by atom from the center of the macrocycle of a single 2H-phthalocyanine. The thus emptied and reactive center is then filled with a silver atom in the second step, which is accomplished by transferring the single atom from the microscope tip. Simultaneously with the metalation process, the resonance frequency change of the oscillating force probe is acquired, which is a measure of the interaction strength. The onset of metalation is signaled by an abrupt decrease of the resonance frequency. The experimental studies reveal that the molecule “pulls” on the silver atom with a force of at least 450 pN, which is associated with an energy for atom transfer of about 275 meV.
Supporting calculations within density functional theory (Prof. Marie-Laure Bocquet, Paris) unveil the mechanism underlying the tip-assisted metalation. The proximity of the tip in the reaction causes a concerted effect where the breaking of the atom-tip bond coincides with the formation of the atom-molecule bond. As a consequence of this effect, the activation energy disappears and leads to a spontaneous atom transfer without further external stimulus, in agreement with the experimental observation.
The study impressively demonstrates the fascinating capability of a state-of-the-art atomic force microscope to follow a chemical reaction between a single atom and molecule with sub-picometer control of the distance between the reactants and to extract important quantitative information about attractive forces at the verge of the reaction. Supporting simulations are important for discovering novel mechanisms. Because of the prototypical character of the experiment – a single atom at the tip, a single molecule adsorbed on a single-crystalline surface – the model calculations rely on a minimal number of assumptions. The presented findings pave the way to the atomically resolved study of chemical reactions at the single-molecule level. In addition to the internationally competitive research competence, the TU Ilmenau offers an excellent technological infrastructure and equipment for this purpose.
Contact
Prof. Dr. Jörg Kröger
Head Group of Experimental Physics I / Surface Physics

