Researchers at TU Ilmenau, together with Danish colleagues, have discovered how an electrical voltage influences the chemical bond between two individual atoms. This made it possible to easily control processes on the atomic scale by means of the macroscopic world. The results of the experimental investigation have just been published in the most important journal for physics research - Physical Review Letters - and were additionally highlighted by the journal's editors through a contribution in "Physics".
In his famous speech "There is plenty of room at the bottom", the famous physicist and Nobel Prize winner Richard Feynman foresaw the great importance of manipulating matter atom by atom. Today, more than sixty years after Feynman's vision, quantum design is being realized in laboratories. This involves forming structures from atomic or molecular building blocks on surfaces that have desired quantum physical properties. The atom-by-atom construction of nano-objects is reserved for modern scanning probe techniques - the scanning tunneling and atomic force microscope - under extreme experimental conditions.
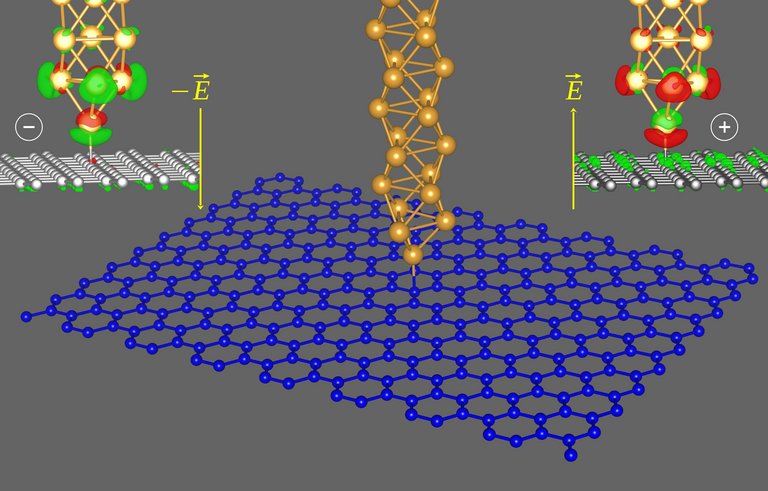
To form such artificial structures requires control over individual atoms on surfaces. In particular, the tip of the microscope must interact temporarily with the atom to be transported. The research team of the Group for Experimental Physics I at the TU Ilmenau, led by Professor Jörg Kröger, has succeeded in realizing this interaction in the form of a chemical bond whose strength can be controlled by means of an applied voltage. For this purpose, the gold atom at the end of the tip of an atomic force microscope and a selected carbon atom in the graphene lattice are brought to a bond distance on a surface with picometer precision. A positive voltage between surface and tip causes a strong gold-carbon bond, strong enough to separate the graphene lattice from the surface by more than 1000 picometers. If, on the other hand, a negative voltage is applied, the bond between the two atoms is too weak to withstand the load.
The physical mechanism underlying the experimental observations has been uncovered by theoretical physicists at the Technical University of Denmark. It turned out to be crucial for the explanation of the effect that the chemical bond character between a gold and a carbon atom is polar. As a result, the bond allows its control by an electric field whose orientation is determined by the polarity of the voltage. The electrons of the bond are shifted more towards the gold or more towards the carbon atom depending on the direction of the field, thus varying the bond strength.
The importance of the work presented in M. Omidian et al, Phys. Rev. Lett. 126, 216801 (2021) lies in the ease of controlling atomic-scale processes by means of the macroscopic world. In addition, this method allows one to manipulate bond strengths so that chemical reactions and catalytic activity can be influenced at the atomic scale. Stackings of 2D materials that feel only weak van der Waals interactions with each other offer a variety of intriguing condensed matter phases determined by the angle of rotation assumed with respect to each other. The new findings will contribute to the fabrication and investigation of such stackings with selectable angles. Experimental physics at TU Ilmenau continues to make progress in the quantum construction of matter and successfully translates Richard Feynman's ideas into new applications.
Contact
Prof. Jörg Kröger
Head Group for Experimental Physics I

